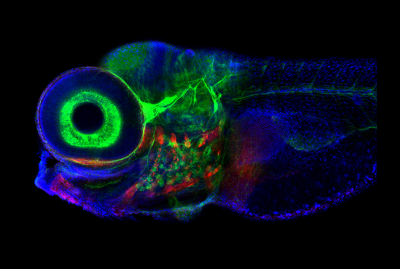
Zebrafish, photographed with confocal microscope. The brain region that controls eye movement is structurally similar in fish and mammals, but the zebrafish system contains only 500 neurons, making it a good model organism. Image credit: Jessica Plavicki
Working with week-old zebrafish larva, researchers at Weill Cornell Medicine and colleagues decoded how the connections formed by a network of neurons in the brainstem guide the fishes’ gaze. The study in Nature Neuroscience found that a simplified artificial circuit, based on the architecture of this neuronal system, can predict activity in the network. In addition to shedding light on how the brain handles short-term memory, the findings could lead to novel approaches for treating eye movement disorders.
Using an array of advanced imaging techniques, the researchers identified the neurons that participate in controlling gaze in young zebrafish, and then determined how these neurons are wired together. They discovered that the system consists of two prominent feedback loops, each containing three clusters of tightly connected cells. The researchers used this distinctive architecture to build a computational model. They found that their artificial network could accurately predict activity patterns of the zebrafish circuit which they validated by comparing their results to physiological data.
Next, the researchers will explore how the cells in each cluster contribute to the behavior of the circuit—and whether the neurons in the different clusters have distinct genetic signatures. Such information could allow clinicians to therapeutically target those cells that may malfunction in eye movement disorders.